To achieve reproducible findings in many enzymatic assays, the substrate and sample must be incubated at specific temperatures without evaporation. If you don’t want to pay thousands for a 96-well plate incubator, than there are two main ways to do this: -The first is preparing 1.5mL Eppendorf tubes and thoroughly vortexing the final solution to […]
Category: Protocols
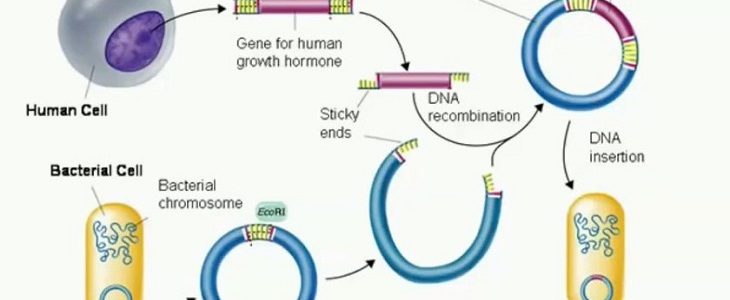
The “Merlin” system for prepping large amounts of recombinant DNA
I always find the innumerable applications for Diatomaceous Earth to be amazing. You keep rocking it little diatoms! In that vein comes a suggestion from Reddit. /u/Tetrazene suggests using the “Merlin” system for prepping large amounts of recombinant DNA: “The buffers are similar to a mini/midi/maxi kit, but they have re-usable fritted columns and use […]
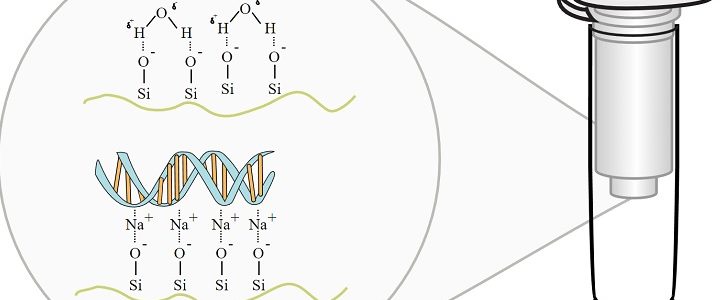
Reusing Nucleic Acid Extraction Columns
If you find yourself trying to find cheaper ways to run mini (or other size…) columns, you should know that you can reuse them without fear of contamination! Nagadenahalli Siddappa, Appukuttan Avinash, Mohanram Venkatramanan, and Udaykumar Ranga describe the process of treating the columns with HCl for reuse in this paper. During Treatment by HCl the “regeneration […]
The Cost of Experimentalists
As a special April Fools post, today we are going to try to discuss the cost of scientists. Specifically, this will be about the cost of scientists in an academic setting. Let’s start with a Post-Doc. In many fields at most universities a Post-Doc is paid based off the NIH/NRSA guidelines. This scales with the […]

Viewing IR Light with Your Phone (Or Laptop)
Traditionally I use this $2000 handled infrared viewer when aligning our Ti:S beam. But recently I’ve been finding that my phone (a Samsung S8) is sensitive to IR light, and that it’s way easier to use than an infrared viewer when aligning the laser. Besides being more flexible with viewing angles (since your face doesn’t […]