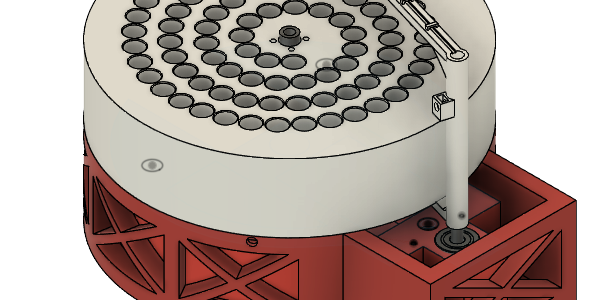
In a follow-up to my all time favorite post, Protein Purification on a Budget: Technical Tuesday, today we are covering a design for a fraction collector for <$100. In their recent preprint, Low-cost, scalable, and automated fluid sampling for fluidics applications , A. S. Boosehaghi, Y.A. Kil, K. H. Min, J. Gehring and L. Pachter […]
Category: Technical Tuesday

Computers on The Cheap
It’s a Technical Tuesday! Today we are going down the rapids of computer purchasing. This is a primer on purchasing computers on the cheap. We start with prebuilt computers (1), then cover acquiring peripherals (2,3), and finally talk about building your own computers (4,5). This is a living document about a volatile topic, so write […]

Technical Tuesday: Penny-Pinching Patterned Projection
Today is Technical Tuesday! A long delayed segment where we try to dive a little deeper into the actual costs involved in particular scientific techniques. We hope to show where you can save money, and what you might lose by saving that money. Today’s topic is dynamically patterning light with Digitial Micromirror Devices. With […]
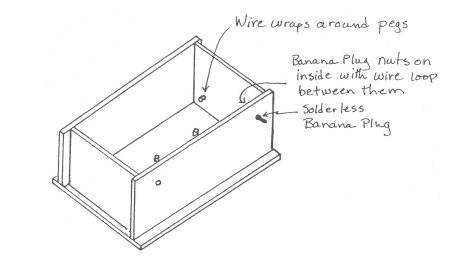
Technical Tuesday: Economical Electrophoresis
Gel Electrophoresis is a method to spatially separate DNA, proteins or macromolecules in general by their weight (or charge). Depending on your field of research you’ve either never heard of this, or they are ubiquitously referred to as “gels” and they are a part of your daily life. The authors of this blog work in a […]

Technical Tuesday: Costs of Cooling
It’s Technical Tuesday! This is a rough one, and we hope people will reach out to us and give us additional advice which we will include here. Today we are talking about the costs involved in keeping things cold, and how to mitigate them. We’ll start with the not very cold, and get colder. But […]